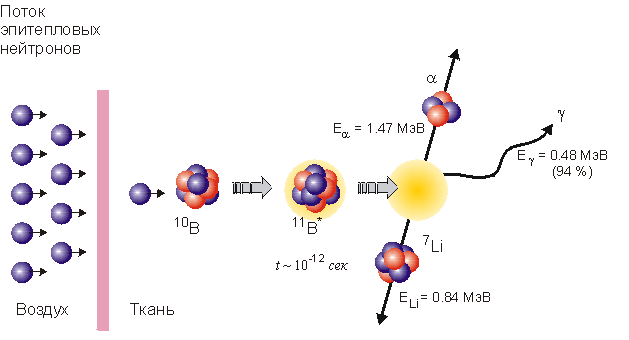
Both a-particles and lithium are high in energy but short in range and high relative biological effectiveness, which means that they destroy the malignant cells in which boron is embedded without hurting the adjacent healthy cells. Therefore, BNCT will make it possible to destroy selectively tumor cells at higher 10B concentration than in normal ones.
In 1951 it was first demonstrated that certain boron compounds would allow higher boron concentration in human brain tumor cells in comparison with normal brain tissue [2]. During 1950-60 at the Brookhaven Medical Research Reactor [3, 4] and Massachusetts Institute of Technology Research Reactor [5] first clinical trials were conducted. Unfortunately, these trials failed to show any evidence of therapeutic effectiveness of the method. Later, it became clear that major reason for their lack of success was low 10B concentration in tumor. Elastic scattering of neutrons and 14N(n,p)14C and 1H(n,g )2H, nuclear reactions resulting in recoil nuclei and g-rays are possible besides nuclear reactions related to neutron capture by boron nuclei at neutron radiation. Although the neutron capture cross-sections for hydrogen and nitrogen are much lower than those for 10B, hydrogen and nitrogen are presented in such high concentrations that their neutron capture "background" contributed significantly to the total absorbed dose.
However, Dr. Hatanaka, a Japanese neurosurgeon who received training with Dr. Sweet at Massachusetts General Hospital at Harvard University, returned to Japan in 1968 and continued to develop the technique [6, 7]. He used new boron drag BSH (Na210B12H11SH, sodium borocaptate) that concentrated selectively in tumor. In this way they began to perform open skull irradiation of brain tumors using thermal neutron beams (energy < 0.025 eV) to reach the target without losing significant amounts of energy. Several groups worked at different reactors using this technique and treated over 200 patients with some encouraging results [6, 7].
At the same time, great progress was achieved in synthesis of boron containing compounds enriched in the 10B isotope. This compound introduced into patient blood produce in a tumor cell the 10B isotope concentration up to 40 mg/g that is three times larger than that in a normal tissue cell. This enables selective destruction of malignant tumors.
The achievements of Dr. Hatanaka inspired researches in neutron capture therapy all over the world. In 1994 BNCT irradiation was re-initiated in the US. Glioblastoma multiforme patients have been treated at Massachusetts Institute of Technology Research Reactor and Brookhaven Medical Research Reactor. In 1997, clinical trials began in Petten, the Netherlands, as a result of joint effort of the European Community. In June 1999 clinical trials began in Finland. Today England, Australia, Argentina, Italy, Germany, Sweden, Slovakia, Czesh Republic, Russia have been settled down to the trials. Extremely encouraging results have been obtained with the treatment of melanoma. Studies currently underway in biological models for other tumors and for non-oncological applications such as rheumatoid arthritis are encouraging and suggest the possibility of new applications of BNCT in the future.
At present, the boron neutron capture therapy is very attractive method for curing malignant tumors, especially therapy for glioblastoma multiforme and melanoma that are resistant to other methods of treatment. Glioblastoma multiforme afflicts approximately one of 20,000 people every year. The disease is always fatal, usually within six months of onset. Surgery and conventional radiation therapies may prolong life for as much as a year but do not stop the spread of tumors throughout the brain. Experiments involving BNCT have tantalized researchers with hints that successful treatment of glioblastoma multiforme is possible.
Progress in BNCT at clinical trials at reactors and prospects of the technique led to intensive discussion of development and construction of neutron source based on compact and inexpensive accelerator available for every oncologic hospital. Various accelerator systems have been proposed to meet the basic requirements of BNCT [8-13]. They include electrostatic quadrupole (ESQ), tandem cascade accelerator (TCA), and radiofrequency quadrupole (RFQ). The ESQ has the disadvantage that the high current H+ ion source is located in the high-voltage terminal of a 2.5 MV dc accelerator, which experience has proven during many years to be very difficult for maintenance and service. The TCA has the advantage that the ion source is located outside the pressure vessel at ground potential and the terminal voltage is only half the beam energy, but it has the disadvantage that beam transmission is limited by ion optics and design of an accelerator tube that can provide vacuum pumping speed and voltage reliability to transport more than 1 or 2 mA of H- ions. The most favorable accelerator that has been examined previously is the RFQ. The RFQ is easy to operate, its ion source is at ground potential and it has low maintenance. However, the RFQ is very expensive and the highest RFQ current available today is up to several mA only.
A new approach in accelerator design is needed to produce the required epithermal neutron spectrum and flux in a reliable compact system and at an acceptable overall cost. The approach we proposed in 1998 [14] is based upon tandem electrostatic accelerator with vacuum insulation and near threshold neutron generation [15, 16]. A high current negative hydrogen ion beam is injected into vacuum insulation tandem. After charge-exchange of negative hydrogen ion to proton inside charge-exchange tube in the center of high-voltage electrode, a proton beam is formed at the outlet of the tandem, which is accelerated to double voltage of high-voltage electrode. Neutron generation is proposed to be carried out by dropping proton beam onto lithium target using 7Li(p,n)7Be threshold reaction. This innovative accelerator which is named vacuum insulation tandem accelerator (VITA) will have two modes of operation. The most efficient innovative operating mode of the facility is at proton energy of 1.889-1.9 MeV that is 10-20 keV higher than the threshold of the 7Li(p,n)7Be reaction. In this mode, neutron beam is provided kinematically collimated in a cone with opening angle of ~25° and average energy of 30 keV, directly applicable for boron neutron capture therapy. The second mode at proton energy of 2.5 MeV will produce a more complex neutron spectrum that extends to 790 keV that may be used directly for the fast neutron therapy or for neutron capture therapy after moderation. Creation of accelerator with proton beam intensity of tens milliamperes will decrease exposure time for necessary therapeutic dose to tens minutes.
If clinical tests show that the unmoderated beam may be used directly, the VITA based facility would provide a compact, low cost solution for BNCT. However, even if a moderator is required, the moderator will be more compact and less expensive because the initial energy spectrum will be less complex and therefore easier to moderate.
It is important to note that the choice of VITA as proton source for BNCT is based upon the need to operate in the threshold region. This is because the operation near threshold demands a highly monochromatic and stable proton beam energy (dE/E = 0.1 %), which rules out the use of an RFQ type accelerator.
[1] G. Locher, Biological Effects and Therapeutic Possibilities of Neutrons, Am. J. Roentgenol. Radium Ther. 36 (1936) 1.
[2]. W. Sweet, M. Javid, The possible Use of Neutron-capturing Isotopes such as boron-10 in the treatment of neoplasms, I. Intracranial Tumors, J. Neurosurg., 9 (1952) 200-209.
[3] L. Farr et al. Neutron Capture Therapy with Boron in the Treatment of Glioblastoma Multiforme, Am. J. Roentgenol. 71 (1954) 279-291.
[4] J. Godwin et al. Pathological study of eight patients with glioblastoma multiforme treated with by neutron capture radiation using boron 10, Cancer (Phila.), 8 (1955) 601-615.
[5] Asbury et al. Neuropathologic Study of Fourteen Cases of Malignant Brain Tumor Treated by Boron-10 Slow Neutron Capture.Therapy, J. Neuropathol. Exp. Neurol. 31 (1972) 278-303.
[6] H. Hatanaka, Clinical results of boron neutron capture therapy. Basic Life Sci 54 (1990) 15-21.
[7] H. Hatanaka, Y. Nakagawa, Clinical results of long-surviving Brain Tumor Patients who underwenr boron neutron capture therapy. Int J Radiat Oncol Biol Phys 28 (1994) 1061-1066.
[8] Yanch et al. Medical Physics 19 (1992) 709.
[9] K. Wang, T. E. Blue, R. A. Gahbauer, Nucl. Technol. 84 (1989) 93.
[10] O. Anderson et al. Proc. 4th Europ. Particle Accelerator Conf., London, June 27 - July 21, 1994.
[11] Proc. 1st Int. Workshop on Accelerator-based Neutron Sources for BNCT. Jackson, Wyoning, USA. CONF-940976, 1994.
[12] Advances in Neutron Capture Therapy. v. 1, Medicine and Physics. Ed. By B.Larsson, Elseiver, 1997.
[13] Application of Accelerators in Research and Industry. Ed. By J. Duggan. AIP Conf. Proc. 392, NY, 1997.
[15] V. Kononov et al. Proc. 2nd All-Union Symp. on use of charged particle accelerator in national economy, Leningrad, USSR, 2 (1976) 60-68 (in Russian).
[16] V. Kononov et al. Proc. 1st Workshop on Accelerator-Based Neutron Sources for BNCT, Sept. 11-14, 1994, Jackson, USA, CONF-94096, v. 2, p. 447. .
 ..
..